We take innovations from one day to day one.
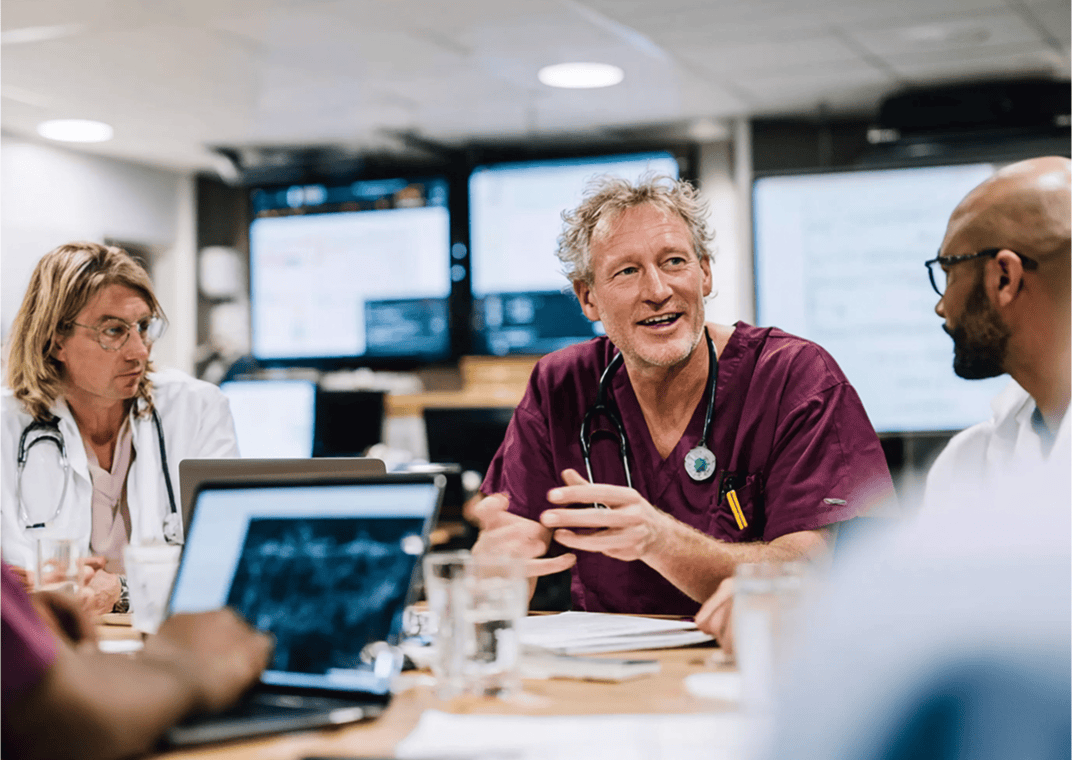
Clinical trials are challenging territory for most innovators. Luckily you don't have to navigate it alone! Mobius is your partner and guide, simplifying the process.
What do you need right now?
Every startup has a different journey. Whether your priority is raising investment, validating feasibility or achieving FDA approval, Mobius builds a pathway tailored to you.

Generate data investors trust
Early feasibility trials in Australia deliver fast, regulator-accepted data that strengthens your pitch.

Plan with clarity and confidence
We map your route from feasibility to pivotal, aligned with FDA, TGA, and ISO 14155 standards.

Build globally accepted evidence
Our ANZ–US bridge model generates data recognised by regulators worldwide.

Accelerate timelines, maintain quality
Local sponsor support and trusted sites cut delays without compromising compliance.

Expert support, streamlined delivery
We handle the complexity so you can focus on innovation and results.
Clinical trials are high risk, high reward.
Poor design can waste data and years of runway. Mobius sets you up for success with globally accepted data and a pathway built for scale.
Avoid delays
Delays burn through limited investor funding. Our process is speedy and cost-efficient.
Full-service model
Clinician-led expertise plus strategic partners, from pre-clinical to post-market.
Globally accepted
If your data doesn't scale, your technology doesn't either. We ensure your data does.
.png)
Efficient trial pathways, scaled.

We simplify complex processes, keep trials inspection-ready, and adapt as your product evolves.”
The Mobius difference
Medical innovations are not one-size-fits-all, so why should your clinical trials be? At Mobius, our team uses their deep expertise to develop a clinical trial strategy tailored for you.
Traditional CRO

More than a CRO. We’re your launchpad.




Guiding you through what you don’t know yet
We answer the questions you may not even know to ask, from readiness to cost, timelines to regulatory differences, so you can move forward with clarity.
If you’ve completed your prototype, generated pre-clinical data based on a robust risk analysis plan, and defined your intended use, you’re likely ready to explore a first-in-human or feasibility study. We can assess your device, documentation, and regulatory pathway to confirm readiness and define the next steps.
Australia offers globally recognised ICH-GCP/ISO 14155-compliant standards, rapid ethics and regulatory approvals, and generous R&D tax incentives—up to 43% cashback on eligible spend. Data collected here are widely accepted by international regulatory authorities, making it the ideal place to start.
Budgets vary by study size, endpoints, and number of sites. Early feasibility studies with full-service typically range from AUD $250K–$1M; pivotal studies are higher. We tailor budgets to your design and help identify tax and funding offsets.
Running early feasibility or safety studies in Australia allows you to collect high-quality data quickly, which can be used in your FDA IDE or 510(k) submission. This “ANZ-to-US bridge” is one of the most time-efficient routes to market.
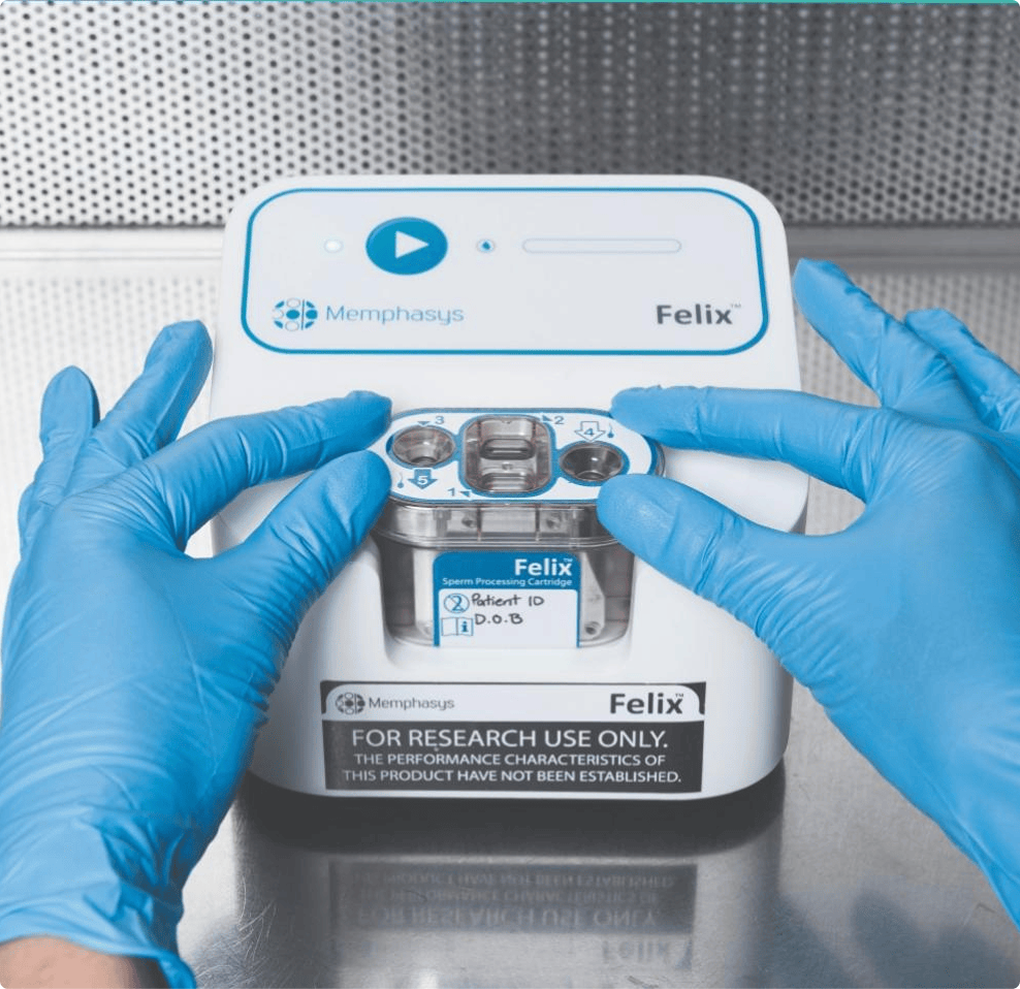
Medical device trials focus on performance and safety, not drug pharmacokinetics. They’re usually smaller, faster, and more iterative—requiring deep technical and engineering understanding as well as clinical rigour. Focus is on safety and feasibility of the application or procedure, technical training and user experience whereas in drug trials, controlled exposure for safety and efficacy are paramount.