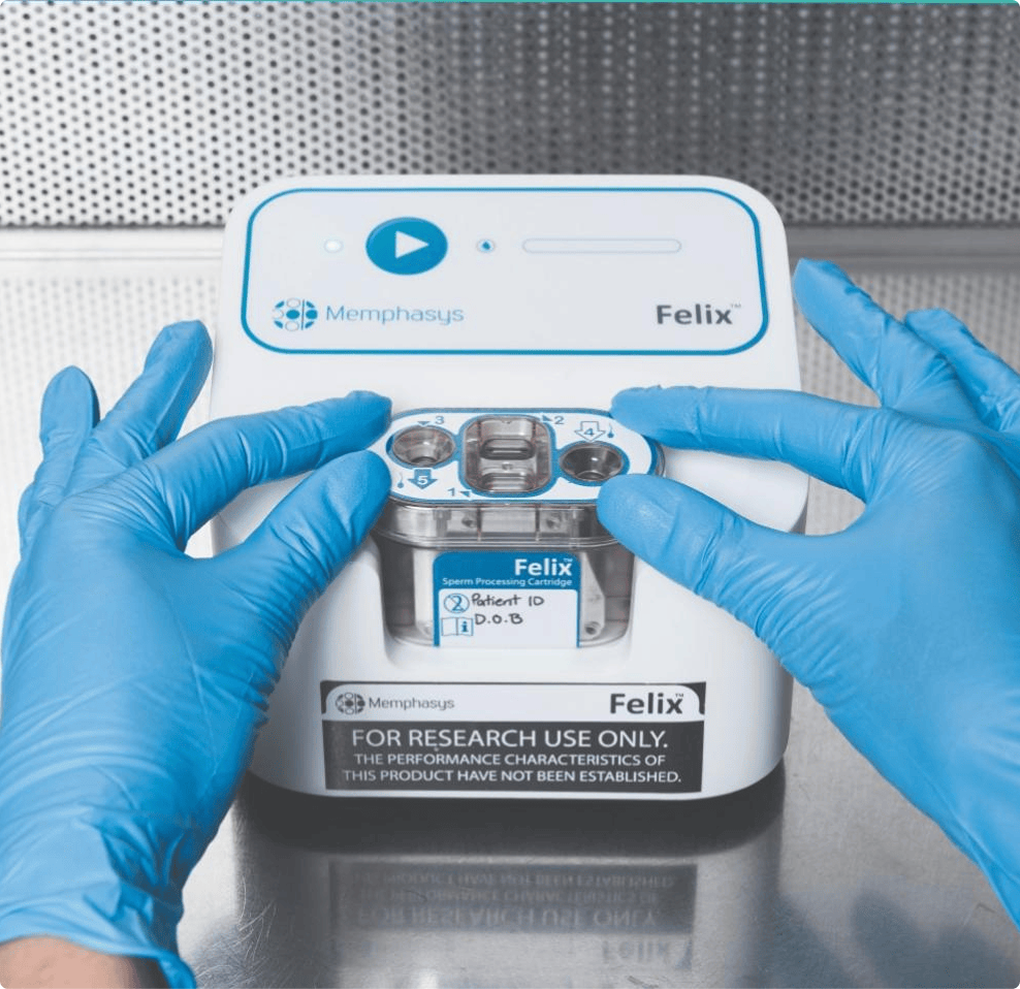
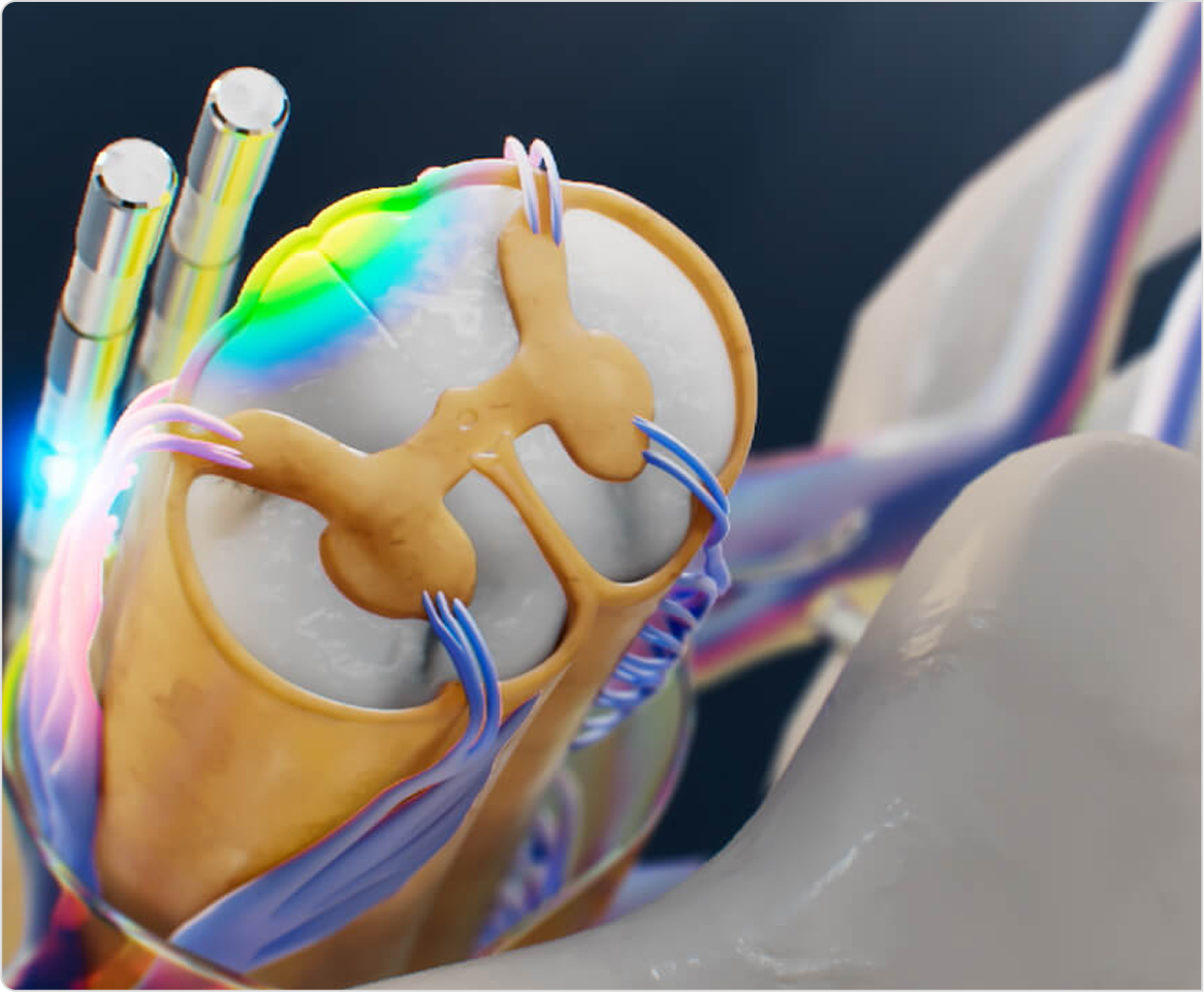
From pathway to proof
Every device has a different pathway but a shared goal - make healthcare better for all involved. Here are some ways we've helped design and execute world-first studies, secure FDA approvals, and transformed healthcare for the better.
Case Studies
Thank you! Your submission has been received!
Oops! Something went wrong while submitting the form.
What partners say about us
Our track record is best told by the founders and clinicians we’ve worked with.
"Mobius Medical have been a pleasure to work with. Their staff are reliable, they have solutions for nearly every query and have a range of experience being able to support from conception through to closeout."

“A great example of the new style of CROs – responsive, easy to work with, cost-effective, collaborative and comprehensive in all their service offerings. We could not have chosen a better partner for our investigative sites.”

"The single most crucial attribute that Mobius Medical provides is their personnel. They have staffed an incredibly skilful, competent and reliable team which is second to none. Enabling them to execute exceptionally well as an organization."