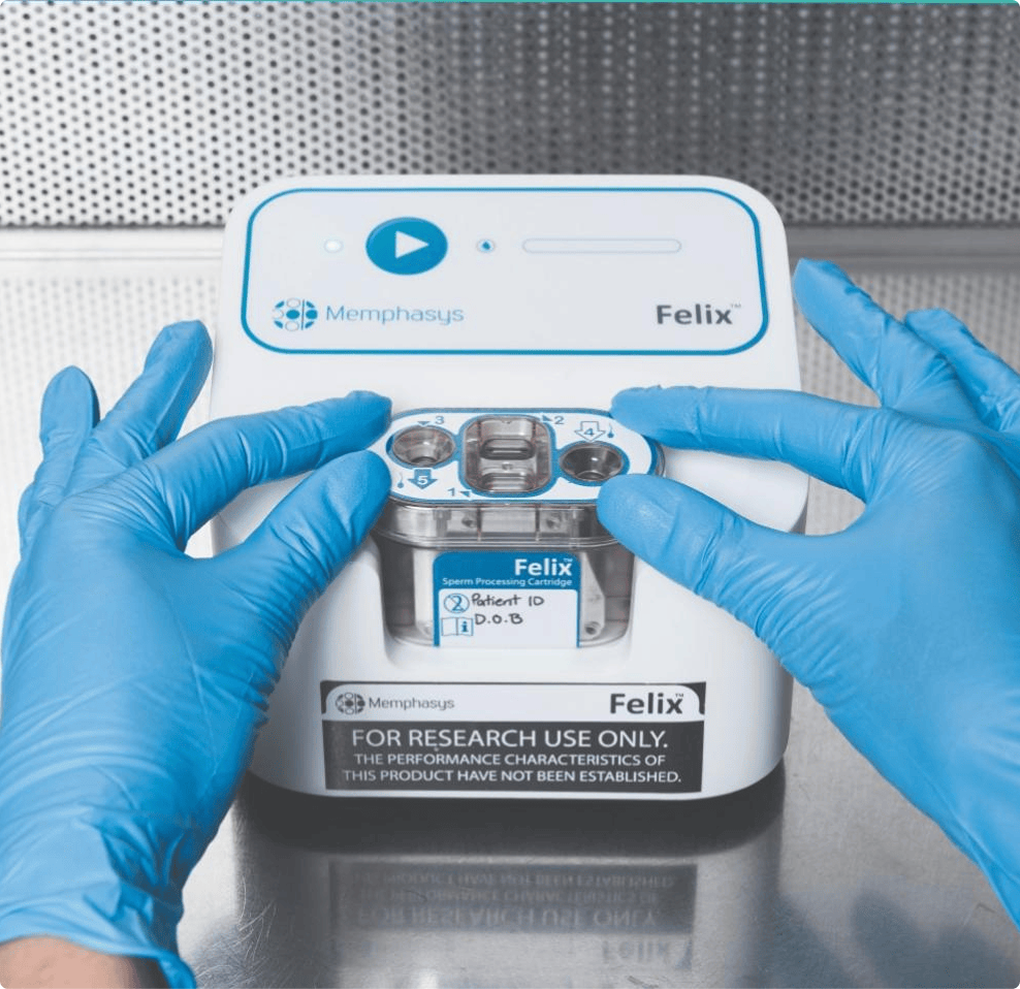
Your innovation, brought to life by our expertise.
We're clinical trial experts with a MedTech edge. Leveraging expertise across Australia, New Zealand, and the USA, we help innovators get off the bench and into the market.
Trusted by MedTech innovators worldwide
Advancing world-first breakthroughs, faster
World first breakthroughs aren't designed to sit on the lab bench forever. They need to be in the hands of doctors and patients - saving lives and transforming outcomes. We help you get there.

Tailored services
Clinical and regulatory strategies that adapt to your pathway
Full-service model
Clinician-led expertise plus strategic partners, from pre-clinical to post-market.

Global standards
Compliance with FDA, EMA, TGA, and other regulatory bodies
The Mobius difference
Medical innovations are not one-size-fits-all, so why should your clinical trials be? At Mobius, our team uses their deep expertise to develop a clinical trial strategy tailored for you.
Traditional CRO